What is Hard Water? |
||
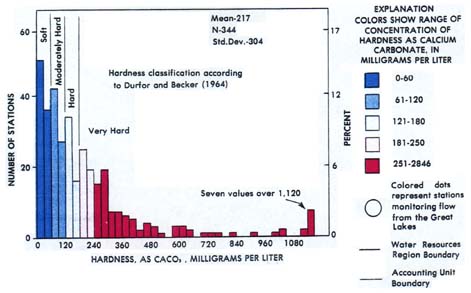
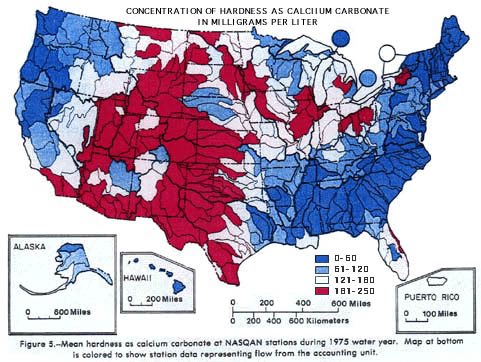
EXPLANATION OF WATER HARDNESS(Provided courtesy of USGS) Many industrial and domestic water users are concerned about the hardness of their water. Hard water requires more soap and synthetic detergents for home laundry and washing, and contributes to scaling in boilers and industrial equipment. Hardness is caused by compounds of calcium and magnesium, and by a variety of other metals. General guidelines for classification of waters are: 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard. Mean values of hardness at 344 stations during the 1975 water year are represented by the chart. The highest 7 values, those over 1,120 mg/L, are lumped in the last bar of the chart in order to maintain the scale. About half of the mean hardness values for the stations are in the soft to moderately hard categories, and about half can be classified as hard to very hard. Patterns of hardness in the United States are shown on the map of accounting units at the bottom of the figure. Softest waters were in parts of the New England, South Atlantic-Gulf, Pacific Northwest, and Hawaii regions. Moderately hard waters were common in many of the rivers of the Tennessee, Great Lakes, Pacific Northwest, and Alaska regions. Hard and very hard waters were found in some of the streams in most of the regions throughout the country. Hardest waters (greater than 1,000 mg/L) were measured in streams in Texas, New Mexico, Kansas, Arizona, and southern California. (From Briggs, J.C., and Ficke, J.F., 1977, Quality of Rivers of the United States, 1975 Water Year--Based on the National Stream Quality Accounting Network (NASQAN): U.S. Geological Survey Open-File Report 78-200, 436 p.)
Maintainer: Office of Water Quality USGS Privacy Statement and Disclaimer URL: http://water.usgs.gov/owq/Explanation.html |
Copyright © 2003-2009 ECMI